GMO Regulations
|
Restrictions on Release into Environment and Maintenance of Lives of GMOs Notification of Certain Releases of GMOs Restrictions on Export of GMOs Intended for Release into Environment Documentation Required for Import and Export of GMOs
|
|
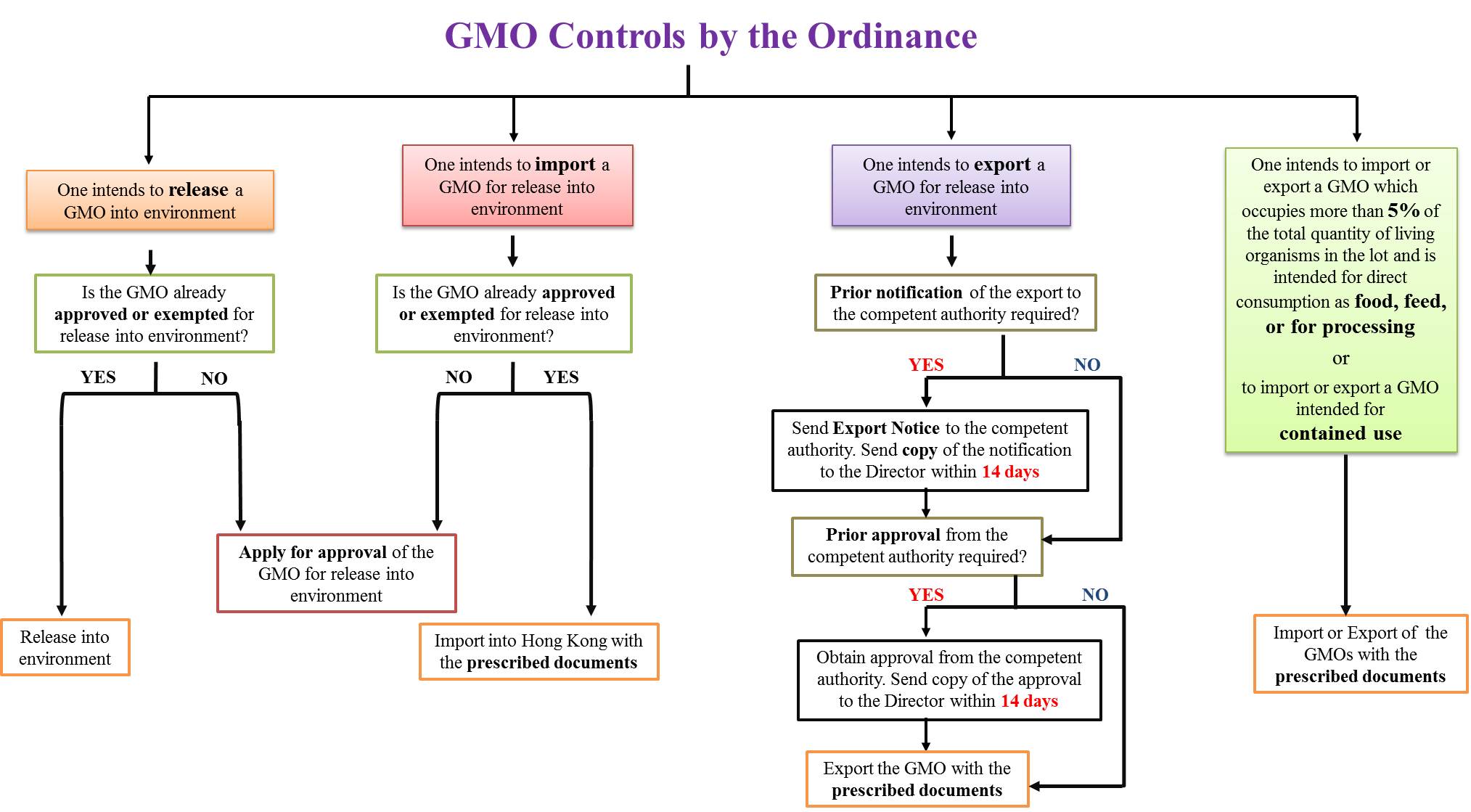
The Genetically Modified Organisms (Control of Release) Ordinance, Cap. 607 (the Ordinance), gives effect to the Cartagena Protocol on Biosafety to the Convention on Biological Diversity to control the release into the environment and the transboundary movement of living genetically modified organisms (GMOs), and provide for related matters. GMOs are regulated according to their intended uses, including:
The Ordinance does not apply to or in relation to a GMO that is a pharmaceutical product for use by human beings. The Ordinance ordains the following controls on GMOs in Hong Kong:
Restrictions on Release into Environment and Maintenance of Lives of GMOs No one is allowed to release a GMO into the environment, import a GMO intended for release into the environment or maintain the life of a GMO that is in a state of being released into the environment, unless:
These restrictions do not apply to or in relation to a GMO that is in transit or transhipment. For detailed approval application process, please refer to the Guidelines for GMO Approval Application. The approval will apply to all subsequent releases, after the GMO is approved and entered into the GMOs Register. There may be conditions attached to the approval. You may browse the GMOs Register for the list of approved or exempted GMOs and the conditions for the approvals or exemption, before making an application or releasing a GMO into the environment of Hong Kong. Contravention to the above restrictions commits an offence and is liable to a fine of HK$100,000 and to imprisonment for one year.
Notification of Certain Releases of GMOs If you have control of a GMO and know that:
you have to inform the Director of Agriculture, Fisheries and Conservation (the Director) of the release by a written notice. The notice must contain the information set out in Parts 1 and 2 of Schedule 1 of the Ordinance. You can use the form Written Notice on Release of GMO for the notification and submit the notice to the Agriculture, Fisheries and Conservation Department. Non-compliance is liable to a fine of HK$50,000 and to imprisonment for 6 months. On receiving the notice, the Director may –
Restrictions on Export of GMOs Intended for Release into Environment This restriction does not apply to a GMO that is in transit or transhipment. To export a GMO that is intended for release into the environment, you have to –
The declarations must be made in the specified forms (Declaration in relation to Export of GMO Intended for Release into Environment). However, requirements (a) and (b) do not apply if prior notification to the competent authority for exporting the GMO to the place is not required under the legal or regulatory requirements of that place. Moreover, requirements (c) and (d) do not apply if prior approval from the competent authority for exporting the GMO to the place is not required under the legal or regulatory requirements of that place. Any person who contravenes requirements (a) or (c) commits an offence and is liable to a fine of HK$100,000 and imprisonment for one year. Any person who contravenes requirements (b) or (d) commits an offence and is liable to a fine of HK$50,000.
Documentation Required for Import and Export of GMOs Shipments containing GMOs (including those intended for release into the environment, contained use and direct consumption as food or feed, or for processing), when being imported or exported, have to be accompanied with prescribed documents to enable easy identification of the GMOs and to provide the contact points for further information. The detailed documentation requirements are laid down in the Genetically Modified Organisms (Documentation for Import and Export) Regulation. It is an offence not to comply with the requirements and is liable to a fine of HK$10,000. The documentation requirements do not apply to shipments containing a GMO-FFP that is unintentionally mixed with other living organisms in the lot and that the percentage of the quantity of the GMO to the total quantity of living organisms in the lot does not exceed the prescribed percentage which is currently set at 5%. More information about the documentation requirements can be found in the Guidelines for Documentation Requirements.
Inspection for the purpose of verifying compliance with various restrictions and requirements under the Ordinance would be taken place on import and export shipments and in or on a place or premises. Inspection of Import and Export Shipments Authorized officers from the Agriculture, Fisheries and Conservation Department would conduct random sampling on import and export shipments for the purpose of verifying compliance with the documentation requirements and the restrictions on import and export GMOs intended for release into environment. The officers may require the person who has control of the shipments to produce the prescribed documents. Samples may also be taken for verification. For import and export shipments of GMOs intended for release into the environment, contravention to section 7 (Restrictions on import of GMOs intended for release into environment) or section 23 (Restrictions on export of GMOs intended for release into environment) of the Ordinance may result in the detention and subsequent forfeiture of the GMOs upon conviction. Inspection of a Place or Premises If an authorized officer reasonably suspects that a GMO is being kept in a place or premises, the officer may, for the purpose of verifying compliance with the restriction on releases into the environment and maintenance of the lives of GMOs, enter and inspect the place or premises during reasonable hours. However, the officer cannot enter and inspect any premises or any part of any premises that is used wholly or principally for dwelling purposes without a warrant. If a magistrate is satisfied by information on oath that there are reasonable grounds to suspect that an offence under the Ordinance has been, is being or is about to be committed in or on a place or premises, or there is evidence of the commission of an offence under the Ordinance in or on a place or premises, a warrant may be issued to authorize an authorized officer to enter and search the place or premises, at the time specified in the warrant or, if no time is specified, at any time. The authorized officer may require the production of, inspect, examine and take copies of any document that is related to compliance with the Ordinance or that relates to the nature or origin of the GMO. The officer may seize, remove and detain any thing that the officer reasonably suspects to be or to contain evidence of the commission of an offence under the Ordinance. Upon conviction of the offence, any thing seized in connection with the offence may be forfeited to the Government. |