|
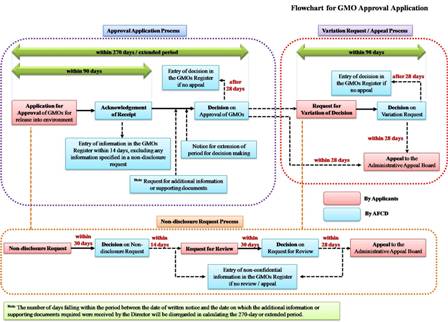
The objective of the Genetically Modified Organisms (Control of Release) Ordinance, Cap. 607 (the Ordinance), is to protect biological diversity from possible adverse impacts arising from the transfer, handling and use of genetically modified organisms (GMOs). Under the Ordinance, prior approval has to be sought from the Director of Agriculture, Fisheries and Conservation (the Director) for release or import for release of GMOs into the environment. The Director will only approve the GMO for release if the possible adverse biosafety effect of the GMO is considered acceptable or manageable. However, it does not apply to or in relation to GMOs that are in transit or transhipment, intended for direct consumption as food or feed, or for processing, or pharmaceutical products for use by human-beings.
The approval application starts with the submission of the GMO approval application form together with the risk assessment report to the Director. At the same time, the applicant may make non-disclosure request on certain part of the submitted information. If aggrieved by the Director’s decision on the GMO approval application or the non-disclosure request, the applicant may request the Director to vary or review his decisions, or lodge an appeal to the Administrative Appeals Board against the Director’s decisions. The applicant may also withdraw by written notice the GMO approval application, variation request, or submitted information and documents.
Application for Approval of GMOs for Release into Environment
The approval for the release of a GMO into the environment applies to all subsequent releases and imports intended for release into the environment after it is entered in the GMOs Register as an approved or exempted GMO. Therefore, before applying for approval, the applicant should browse the GMOs Register for the list of approved or exempted GMOs and the conditions for their approval or exemptions. The process of the approval application is described below:
-
Approval Application
The GMO approval application should comprise:
-
-
a report on a risk assessment carried out, or caused to be carried out, by the applicant in accordance with Schedule 3 of the Ordinance on the possible adverse biosafety effect of the GMO, which should also include:
-
a general description of the GMO;
-
risk assessment on the possible adverse biosafety effect of the GMO; and
-
any proposed methods and plans for dealing with the possible adverse biosafety effect of the GMO in emergency circumstances.
The risk assessment report must be carried out in a scientifically sound and transparent manner, and may take into account expert advice of, and guidelines developed by, relevant international organizations.
The prescribed fee payable for the GMO approval application is HK$14,250, which should be in cheque, cashier order or via online payment and made payable to “The Government of the Hong Kong Special Administrative Region”. The applicant has to submit the GMO approval application in person, by post or by email to Biodiversity Conservation Division, Agriculture, Fisheries and Conservation Department, 6/F, Cheung Sha Wan Government Offices, 303 Cheung Sha Wan Road, Kowloon, Hong Kong.
-
Acknowledgement of Receipt of GMO Approval Applications
Within 90 days after receiving the GMO approval application, the applicant will be issued a written acknowledgement from the Director confirming the receipt of the application. The acknowledgement will state the date on which the Director received the application and whether the application contains, on the face of it, all the necessary information.
We are committed that the acknowledgement of receipt of GMO approval application will be issued to the applicant in an expeditiously manner.
-
Making Decision
After receiving the GMO approval application, the Director will make a decision on the application within 270 days. But the period may be extended with a written notice, which will state the duration of the extended period and the reason for the extension.
Meanwhile, the Director may, by written notice, require the applicant to provide additional information or supporting documents, or to appear before the Director to provide any clarification or answer any question raised. The period from the date on the written notice to the date on which the additional information or supporting documents required is received by the Director, will be disregarded from the 270-day or the extended period.
-
Decision Notice
Before the expiry of the 270-day or extended period, the applicant will be informed of the decision on the approval of the GMO application by written notice. The approval may be given with or without condition. If the application is refused or a condition is attached to an approval, reason will be given in the written notice.
Non-Disclosure Request
The Director will enter all information submitted for the purpose of GMO approval application or variation request, in the GMOs Register within 14 days after receipt of it. When providing any information to the Director for the purposes of a GMO approval application or variation request, the applicant may request the Director not to enter specified information in the GMOs Register.
-
Non-Disclosure Request
The request must be made in the specified form (Request Form for Non-disclosure or Review of Decision on Non-disclosure), which specifies the information that should be kept confidential and the justifications for the request. The non-disclosure request should be submitted at the same time as the approval application. However, the request must not be related to the following information:
-
the name and address of the applicant;
-
a general description of the GMO;
-
a summary of the risk assessment on the possible adverse biosafety effect of the GMO; and
-
any proposed methods and plans for dealing with the possible adverse biosafety effect of the GMO in emergency circumstances.
-
Decision Notice
Within 30 days after receiving a non-disclosure request, the Director will decide that none, part or all of the proposed confidential information is to be entered into the GMOs Register. The applicant will be informed of the decision and the reason for it by a written notice.
Request for Variation or Review of Director's Decisions
-
Request for Variation of Decisions on GMO Approval Application
The applicant may apply for variation of decision on a GMO approval application or to cancel or vary the conditions attached to the release, if there is:
-
a change in circumstances that may influence the Director’s assessment on the possible adverse biosafety effect of the GMO;
-
additional scientific or technical information that may influence the Director’s assessment on the possible adverse biosafety effect of the GMO.
The request must be made in the specified form (Request Form for Variation of Decision on GMO Approval Application) accompanied by the relevant information in support of the request and the prescribed fee payable (HK$1,890). Within 90 days after receiving a variation request, the Director will decide whether to confirm or vary the decision. The applicant will be informed of the decision and the reason by a written notice.
-
Request for Review of Decisions on non-Disclosure Requests
The applicant may apply for review of decisions on non-disclosure request. The request must be made in the specified form (Request Form for Non-disclosure or Review of Decision on Non-disclosure) accompanied by the justifications for the request and the prescribed fee payable (HK$1,010).
Within 30 days after receiving a variation request, the Director will decide on the request. The applicant will be informed of the decision and the reason by a written notice.
Appeal to the Administrative Appeals Board
Within 28 days after receiving notice of the decision, the applicant may appeal to the Administrative Appeals Board against the Director’s decisions on GMO approval applications, requests for variation of decisions on GMO approval application and requests for review of decisions on non-disclosure requests.
Withdrawal
-
Withdrawal of GMO Approval Applications or Variation Requests
The applicant may, in writing, withdraw a GMO approval application or a request for variation of decisions on GMO approval application at any time before the Director makes the decision. In such case, the process of the application or request will cease. However, all fees under this Ordinance, including the application fee for approval of GMO for release into environment, are non-refundable.
-
Withdrawal of Information or Documents Provided
The applicant may, in writing, withdraw any information or documents provided for the purposes of an application or request at any time before the Director makes a decision. In such case, the process of the application or request will continue as if the information or document had not been provided.
Safe Transport and Storage
GMOs to be transported and stored for release into the environment should be kept in a safe place and appropriate measures should be imposed (e.g. being contained inside a sealed, unbreakable container) to avoid spillage, leakage or escape of the GMOs. The shipment containing the GMOs should be labelled for easy identification. In case of unintentional release, the GMOs must be located and recovered or rendered non-viable. Incidents of accidental release (whether recovered and disposed or not) should be reported to Agriculture, Fisheries and Conservation Department as soon as possible.
Warning
If the GMO is NOT:
-
an approved GMO and any condition for the approval of the GMO, as set out in the GMOs Register, has been complied with; or
-
an exempted GMO and any condition for the exemption of the GMO, as set out in the GMOs Register, has been complied with,
a person who knowingly:
-
releases the GMO to the environment;
-
imports the GMO which is intended to be released into the environment; or
-
maintains the life of the GMO that is in a state of being released into the environment,
commits an offence and is liable to a fine of HK$100,000 and to imprisonment for one year.
|
